Scientists have long known that a diverse selection of microbes reside in our bodies. While most live in the large and small intestines, they are present throughout the body – including the vagina. The uterus (womb), however, has long been assumed to be a sterile environment with the assumption that the presence of bacteria indicates a diseased state. As such, there has been a popular belief that newborn babies are born sterile and are colonized only during and after delivery.
This view is no longer uniformly accepted as recent research reveals the presence of microbes not only within the uterus but in other pregnancy-related tissues e.g. amniotic fluid and placenta. As such, we must consider how these microbes relate to women’s health as well as how this impacts the development of the newborn microbiome.
The vagino-uterine microbiome
So, firstly, how was it found that the environment within the uterus isn’t sterile? Observations from both healthy pregnancies and relevant animal model studies indicate that the foetus may first be exposed to bacteria during gestation, the time between conception and birth.
Indeed, recent studies of reproductive-age women have found that the reproductive tract contains microbes throughout, with a continuous stream of microbes from the vagina (A) and cervix (B) through to the endometrial lining (C), uterus (D) and fallopian tubes (E). This has been shown to be true both before and during pregnancy. This is what is known as the vagino-uterine microbiome.
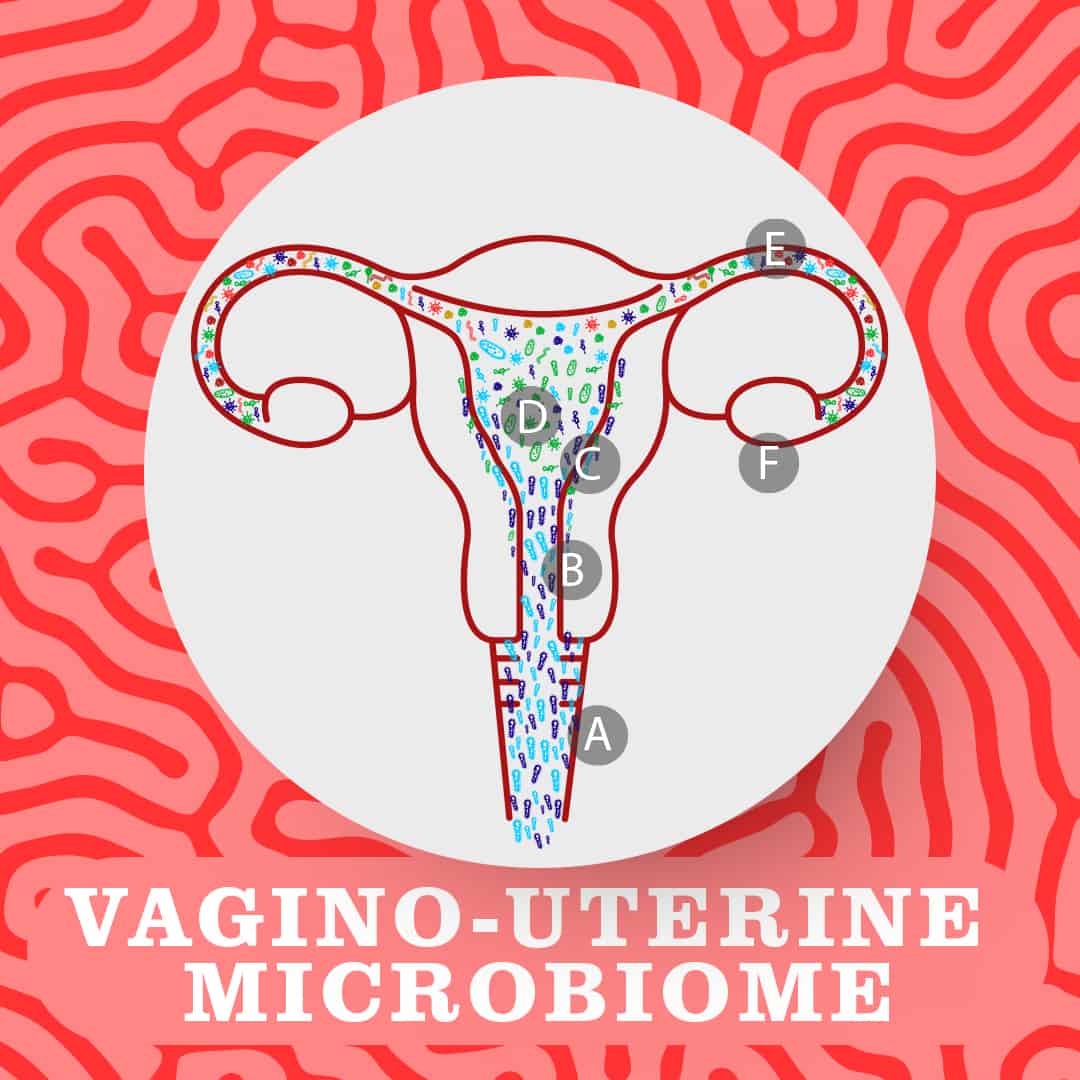 A key study published in Nature in 2017 identified distinct microbial communities in these different areas. Notably, the lower reproductive tract (the vagina and cervix) exhibits low bacterial diversity and is dominated by Lactobacillus bacteria, while the upper reproductive tract (the uterus and fallopian tubes) is much more diverse with a variety of microbial species growing there. Interestingly, the researchers found correlation between differences in these microbes and the different phases of the menstrual cycle.
A key study published in Nature in 2017 identified distinct microbial communities in these different areas. Notably, the lower reproductive tract (the vagina and cervix) exhibits low bacterial diversity and is dominated by Lactobacillus bacteria, while the upper reproductive tract (the uterus and fallopian tubes) is much more diverse with a variety of microbial species growing there. Interestingly, the researchers found correlation between differences in these microbes and the different phases of the menstrual cycle.
It has been suggested that examining the vagino-uterine microbiome could have a role in the detection of common reproductive diseases (such as adenomyosis and endometriosis). Some researchers have even suggested a link between the microbiome and endometrial cancer.
Transfer of microbiota from mother to foetus
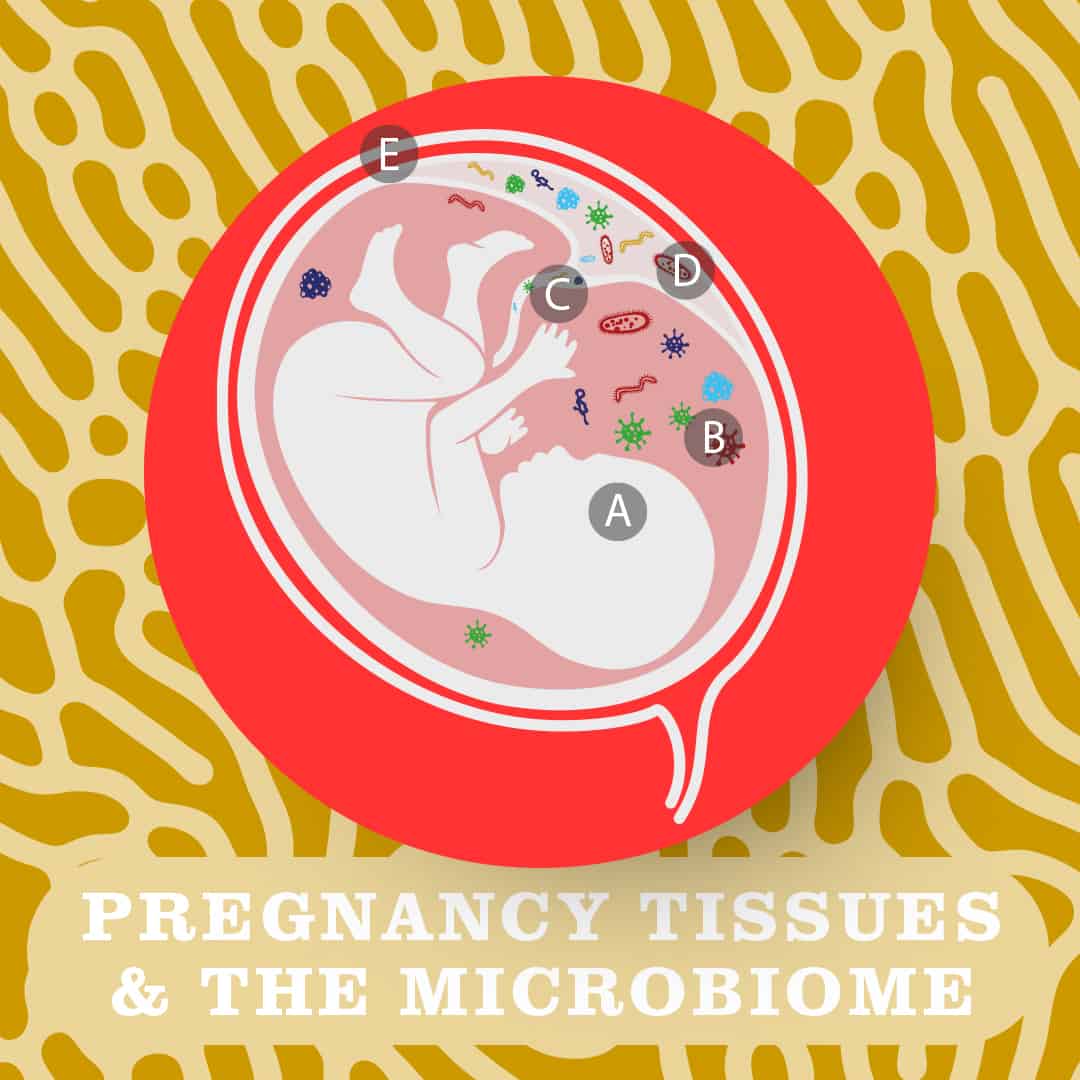
The presence of commensal microbes is not thought to negatively impact the pregnancy or health of the growing foetus (A) as bacteria have been detected in the foetal membranes (e.g. amnion and the chorion, which make up the protective amniotic sac (B) and in umbilical cord blood (C) of healthy, term pregnancies. Bacteria have also been detected in placenta (D). (E depicts the uterus)
Examination of 320 placentae found that the most common group of bacteria were Proteobacteria, which includes bacteria such as Escherichia coli (E. coli), as well as Fusobacterium and Streptococcus species – all of which are also common to the oral cavity. It should be noted that the abundance of microbes in placenta is low but the bacteria present are diverse and metabolically rich.
It is likely that microbes are transferred into the foetus across the maternal-foetal interface via the placenta, as the microbial makeup of the meconium (the earliest excretion by an infant) and placenta were shown to be significantly similar.
Multiple animal models have allowed scientists to identify bacteria from the foetal intestine which has provided further evidence of microbiota transferal from mother to offspring prior to delivery (though the precise route by which this occurs is unclear).
Early life & the neonatal microbiome
Many factors can be considered that may influence the development of the microbiome of the newborn infant (neonate). Below, we take a closer look at 3 such factors.
- Preterm birth

Births can be classified depending on gestational age at the time of birth as either preterm, full term, or postterm. A baby is considered full term if born between 37 and 42 weeks of age; birth before 37 weeks is considered preterm, and after 42 weeks considered postterm.
It has been shown that the microbiome of a newborn differs between infants born at full term and those born prematurely (preterm). Two key differences have been noted:
Firstly, the microbiome of a preterm infant tends to be much more sparsely populated, with a delay in colonization of healthy (commensal) bacteria (e.g. Bifidobacterium).
At the same time, the microbiome of a preterm newborn contains higher quantities of harmful (pathogenic) bacteria (e.g. Klebsiella pneumonia, Clostridium difficile) than term newborns.
A preterm birth, and the underlying factors leading to it, impact the short and long term outcomes of newborns. Risk of infection, intestinal problems and other diseases is heightened in preterm infants due to insufficient development of both the infant’s body tissues and also its immune regulation (reducing its ability to fight infection). The increase in pathogenic bacteria resulting from lower gestational age at birth is also thought to play a role.
There is uncertainty as to what degree the prebirth underlies microbial differences observed as opposed to changes to postnatal care which expose the infant to different environmental factors (antibiotic use, sterile isolators and other factors associated with care in a neonatal intensive care unit (NICU)).
Further, whether the higher abundance of pathogenic bacteria are causally related to preterm labor and birth remains unknown.
- Breastfeeding

Like the uterus, it was once thought that human breast milk is sterile. We know now that this is far from true. Breast milk is a highly complex source of nutrients which has its own distinct microbiome filled with a variety of bacterial species. In fact, breast milk contains a mix of bacteria associated with other areas of the body – skin-associated Staphylococcus, oral-associated Streptococcus, and even gut-associated Bifidobacterium and Enterococcus.
How the latter bacteria get from the gut to the mammary glands (which produce breast milk) is particularly intriguing and suggests a unique pathway that is aided by the increased blood/lymph supply to the breasts during pregnancy. This hypothesis is supported by a study which showed that mothers taking oral Lactobacillus probiotics were then found to have the same strains in their breast milk (in 6 out of 10 cases looked at).
It should be noted that human milk contains many components beyond bacteria that are transmitted to offspring and the diet of a mother can affect breast milk and, as a result, the infant gut microbiome.
- Mode of delivery
There are two methods of delivery by which women give birth; vaginally and surgically via cesarean delivery (“C-section”).
Concerns of increased disease risk due to lack of exposure to the vaginal microbiome through C-section has led to the unorthodox and not clinically-recommended post-birth practice known as ‘vaginal seeding’. This entails swabbing the face of a baby with a swab used on the mother’s vagina in the hope of transferring bacteria they would otherwise be exposed to during delivery.
However, there is considerable evidence that indicates the newborn microbiome is not significantly altered by vaginal delivery:
Firstly, the vaginal microbiome is dominated by Lactobacillus species, which are not dominant in the newborn microbiome and have been shown to rarely transfer to the newborn during vaginal delivery.
Secondly, the origin of many bacteria in newborns has been shown to be from the maternal gut microbiome – to which both infants born by vaginal and C-section are exposed.
This is not to say that the microbiome of newborns born by either mode of delivery do not differ. A large scale study evidenced distinct microbiome profiles for newborns born via C-section in the first week of life compared to those born via vaginal delivery – but this was short-lived and did not last past 2 months of age. Indeed, by 6 weeks, no differences were identified between either cohort of infants.
So, what is causing this difference?

It is uncertain whether early differences are due to mode of delivery directly or rather due to underlying conditions associated with C-section (though this can also be a voluntary choice without complications) or even differences in postnatal care.
Not all factors have been extensively researched but, a notable 2019 large-scale study found that differences in the gut microbiomes of vaginal-delivered and C-section -delivered babies was due to hospital-acquired bacteria – found in greater quantities in a larger proportion of babies born by C-section.
Beyond this, maternal diet and formula feeding (vs breast feeding) appear to have a longer lasting (beyond 6 weeks) effect on the infant microbiome.
Overall, it’s clear that the maternal microbiome has an impact on the microbiome of an infant, though through development in the uterus and exposure to gut microbes rather than through vaginal deliver as once thought. In contrast, how birth mode influences the risk factors for disease through influence of the microbiome remains unclear. But what we do know is that multiple factors such as gestational age and birth and maternal diet do have a lasting impact on the developing human microbiome.
Want to know more? Keep your eyes peeled for our upcoming part 2. In the meantime, browse our Content Hub for similar content and follow our Instagram account to receive the latest updates.
References:
Chu et al, 2019 – Gastroenterology Clinics of North America
Shao et al, 2019 – Nature
Chen et al, 2017 – Nature Communications
Kwon, 2017 – The Scientist
Quinn et al, 2016 – Vaccine
Rodriguez, 2014 – Advances in Nutrition
